When two different metals are coupled together in atmosphere or water, the likelihood of developing galvanic corrosion can be predicted using a “galvanic series.”

When two different metals or alloys are immersed in a corrosive solution or regularly connected by moisture, each will develop a corrosion potential. If the conditions for galvanic corrosion are present, the more noble metal will become the cathode and the more active metal will become the anode. A measurable current may flow between the anode and the cathode. If this occurs, the anode’s rate of corrosion in the service environment will be increased while the cathode’s corrosion rate will decrease. The increased corrosion of the anode is called “galvanic corrosion.”
Galvanic corrosion is sometimes used to extend the life of materials (i.e. zinc coatings on carbon steel and zinc anodes in water heaters), but, if it is not considered and the right conditions exist, it can lead to unexpected failures.
Requirements for Galvanic Corrosion:
In order for galvanic corrosion to occur, three elements are required.
If any of these elements is missing, galvanic corrosion cannot occur. If, for example, the direct contact between the two metals is prevented (plastic washer, paint film etc.) or if there is some other interruption in the conductive path, there cannot be galvanic corrosion and each metal will corrode at its normal rate in that service environment. Figure 1 shows examples of conditions that do not meet all requirements for galvanic corrosion.
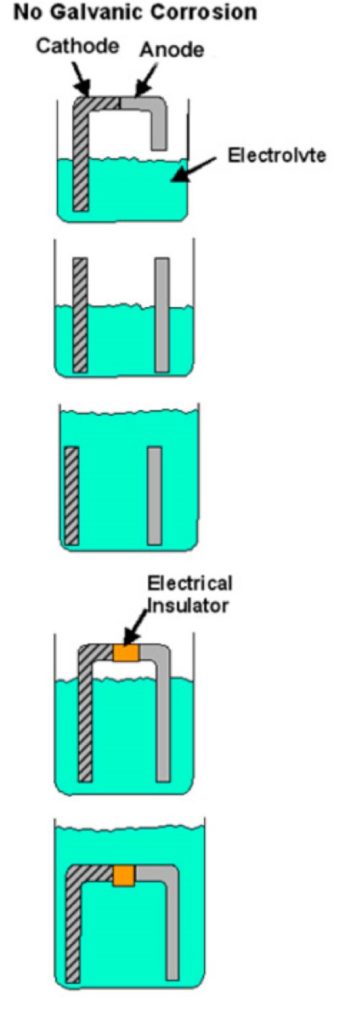
Examples of bi-metallic combinations when galvanic corrosion cannot occur
When two different metals are coupled together in atmosphere or water, the likelihood of developing galvanic corrosion can be predicted using a “galvanic series.” In specialized applications, such as when dissimilar metals are embedded in concrete, corrosion data for that specific environment should be used.
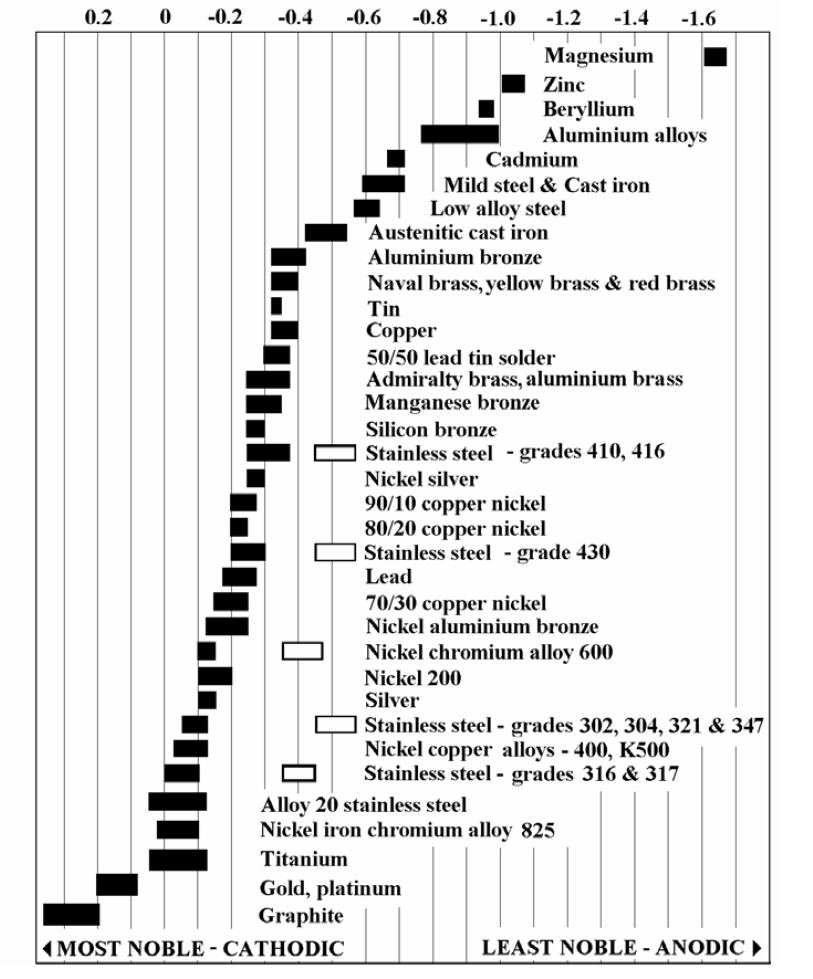
Figure 2 shows the galvanic series measured in seawater for some common metals and alloys. When two metals are further apart in the list (e.g. a larger difference between the two numbers), the driving force for galvanic corrosion is increased. The most anodic (active) metals are at the top and most cathodic (noble) at the bottom. Both solid and hollow bars are shown for the stainless steels. The hollow bars represent actively corroding stainless steel, which has a different potential then passive (not corroding) stainless steel. In most applications, where dissimilar metals are combined, the passive (solid) bar should be used to determine the position of the stainless steel.
For example, if zinc (think galvanized steel) which is an active material and near the top of the list and stainless steel, a noble metal and near the bottom of the list were in direct contact and in the presence of an electrolyte (water), galvanic corrosion will occur if they are regularly exposed to an electrolyte.
In addition to the three elements sighted above, the relative surface area (not mass) of each of the exposed metals is also an important factor. (See Figure 3). If the area of the cathode (noble metal) is very large, and the anode (active metal) is very small, the current produced is likely to be very high and the anode will corrode quickly.
For example, if a window frame made of stainless steel and it is attached with carbon steel screws, the screws will probably corrode at an accelerated rate. If the area of the cathode (noble metal – stainless steel) is very small, and the anode (active metal – carbon steel) is very large, the current produced will be very low and the corrosion rate of the anode may not be affected. If the window frame is made of carbon steel and it is attached with stainless steel screws there will be very little, if any, galvanic corrosion.
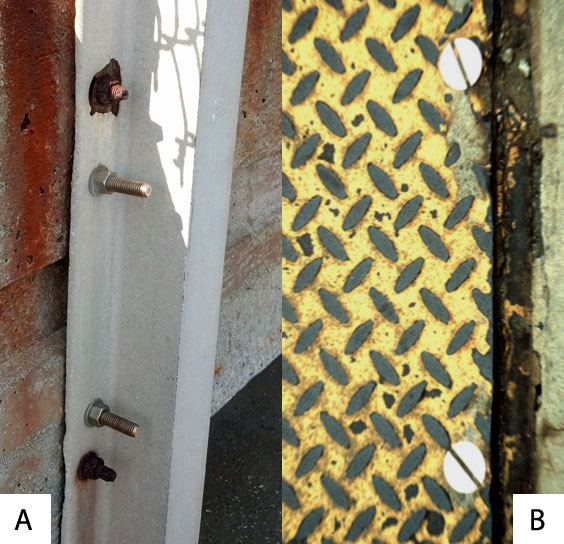
Figure 3A shows the galvanic corrosion of carbon steel bolts used to secure a stainless steel structural railing support on a bridge. The small surface area of the active bolts results in an undesirable galvanic couple and they are exhibiting an accelerated corrosion rate. Image 3B shows stainless steel fasteners used to secure a carbon steel tread plate. The relatively small surface area of the stainless steel fasteners means that they have essentially no galvanic effect on the corrosion rate of the carbon steel plate.
Figure 3: Examples of good and bad galvanic corrosion ratios
Dissimilar metal combinations should be avoided in areas where moisture is likely to accumulate and remain for long periods. In well-drained exterior applications, dissimilar metals can be used together if favorable surface ratios exist, but the best solution is to electrically insulate one from the other. When painted carbon steel and stainless steel are welded together, the welded joint should be painted. Stainless steel fasteners with neoprene or other inert washers are regularly used with other metals.
Research has shown that galvanic corrosion is not a concern between stainless and carbon steel in concrete.

The Statue of Liberty (completed in 1886) is one of the highest profile examples of the damage that galvanic corrosion can cause. The original design used a copper exterior skin (large cathodic or noble surface area) supported by a cast-iron structural frame (small anodic or active surface area) with the metals separated by wool felt which eventually failed. In 1984, it was closed to the public due to significant corrosion of the cast-iron frame. It was rebuilt using a duplex stainless steel structural frame. Copper alloys and stainless steels are quite close in the galvanic series with the duplex being more cathodic which is appropriate since it has the smaller surface area ratio.
Aluminum adjoins zinc in the galvanic series. Decision makers are often aware that aluminum’s corrosion rate in atmosphere is between that of carbon steel and stainless steel, but, when it is directly coupled to another metal and an electrolyte is present on a regular basis, it becomes very anodic (active) and it will corrode at a higher rate than either carbon steel or stainless steel which are both more cathodic (noble). For that reason, if aluminum structural framing is used to support sheets of stainless steel and they are in direct contact with moisture present, the aluminum’s corrosion rate can be accelerated.
When multiple metals must be combined in direct contact and an electrolyte is likely to be present, the fasteners should always be specified to match the noblest of the metals being joined.
Not all environments produce the same galvanic results. Research has shown that galvanic corrosion is not a concern between stainless and carbon steel in concrete.